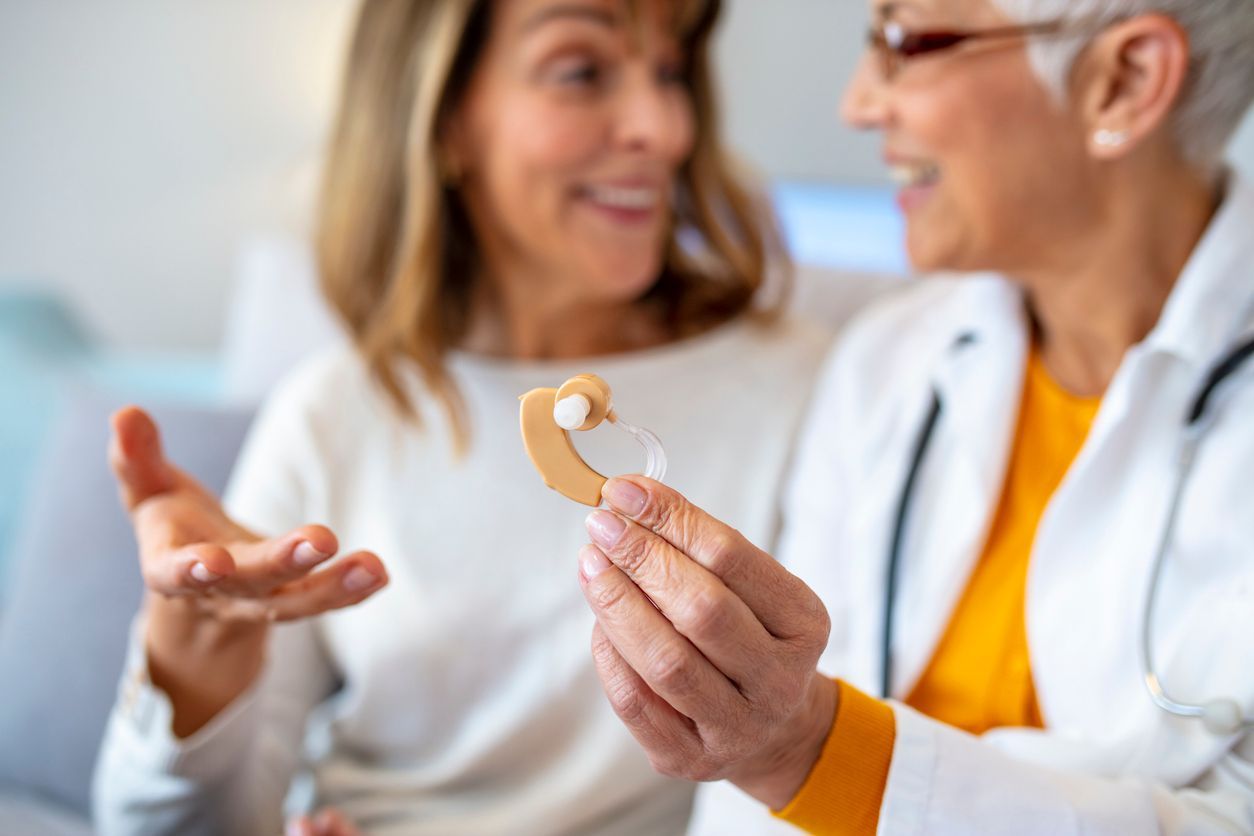
Retail Stores

You have seen them—the endcap in the local discount or drug store with devices that are labeled as “hearing aids.” The price might range anywhere from $49.99 for a very basic sound amplifier to $899.00 for a product that is now classified by the FDA as an over-the-counter hearing aid. Despite the price point, they often all reside in the same display case, all posing as the same types of devices you would get at a private audiology practice. These devices may have a label that uses language like FDA Registered or FDA Certified with the FDA logo on the box. Is that the same thing as FDA approved, FDA cleared, or FDA authorized? The short answer is NO. There might be a host of monikers on devices, and even the devices that now fit into the FDA guidelines that were released in 2022 for over-the-counter hearings aids, all such products need to be examined carefully before you spend hard earned money on devices that will give you little benefit. For information on devices that do not fall under the new FDA guidelines, read on for additional information.
What does it mean when something is FDA Registered?
According to the FDA, “owners or operators of places of business that are involved in the production and distribution of medical devices intended for use in the United States are generally required to register annually with the FDA.”
It's important to understand that when a facility registers its establishment and/or lists its devices, it is simply that, a registration. The FDA requires this of all such businesses so that they know what businesses are operating in the U.S. and what products they are making and selling. The resulting entry in the FDA’s registration and listing database DOES NOT IN ANY WAY denote approval, clearance, or authorization of that facility or its medical devices.
Are there FDA Certificates? No.
Some retail store-based hearing aids will even include an FDA certificate in its packaging. What does this mean? When a business involved in the production and distribution of medical devices intended for use in the United States registers with the FDA, they do not receive a certificate from the FDA.
It's important to understand that the FDA DOES NOT ISSUE ANY TYPE of device registration certificates to medical device facilities. In addition, the FDA does not “certify” registration information for businesses that have registered and listed.
Misleading FDA Registration Certificates
Some firms sell medical devices in the United States alongside "FDA registration certificates," such as the sample certificate depicted here.
Example of a Fraudulent Certificate
These certificates often have the look of an official government document and may include the FDA logo. However, FDA does not issue device registration certificates.
Firms that misleadingly display certificates alongside information about and photos of a device for sale in the United States to imply review or approval by FDA of the device misbrand the device in violation of the Federal Food, Drug, and Cosmetic Act.
How Do You Know if the FDA Approved, Cleared, or Authorized a Medical Device?
The FDA provides several ways for you to check if the FDA approved or cleared a medical device or, as described below, if the FDA authorized the device to be used during a public health emergency.
You can search for FDA-approved or FDA-cleared products by device name or company name:
Go to the Devices@FDA Database.
In the Enter a search term in the space below field, type the name of the device or the company name. You can type the exact name of a specific device or a generic name for a category of devices (such as hearing aid or cochlear implant.
Click Search.
It is important to understand that a true hearing aid is a class II medical device and MUST be FDA approved and then programmed specifically for you based on the results obtained during a comprehensive hearing evaluation at a licensed audiology practice, ENT office or licensed hearing aid dispenser.
Even if a hearing aid purchased at a discount or drug store has instructions for taking an online hearing test at home and then emailing the results for “programming” by a “trained professional” this type of fitting of a hearing aid is inferior, at best, to the professional care you will get from a university-trained audiologist and is a danger, at worst, since improper amplification can result in damage to your ears. Additionally, hearing loss may result from a medical condition like a bacterial or viral infections or a tumor. By self-treating for hearing loss, these medical conditions go undetected.
Bear in mind, if a company resorts to presenting itself as something it’s not by slapping an FDA logo on the packaging and presenting misleading labels and wording, you should beware of the product.
What about the Over-the-Counter hearing aid designation that went into effect in 2022
Over-the-counter hearing aids are a new official designation that the FDA released official guidelines on in August 2022.
Recent Posts